STUDY DESIGNS
FUTURE 21-5
FUTURE 2 was a phase 3, multicenter, randomized, double-blind, placebo-controlled trial that is evaluating 397 adult patients with active PsA (≥3 swollen joints and ≥3 tender joints) despite use of NSAlDs, corticosteroids, or DMARDs. Patients had a diagnosis for ≥5 years and were randomized in a 1:1:1 ratio to receive COSENTYX.
150 mg (n=100), 300 mg (n=100), or placebo (n=98) subcutaneously at Weeks 0, 1, 2, 3, and 4, followed by the same dose every 4 weeks. Patients who received placebo were rerandomized (1:1) to COSENTYX 150 mg or 300 mg every 4 weeks, at Week 16 or Week 24, based on responder status. The primary end point was the percentage of patients with ACR20 response at Week 24.2
After Week 24, patients knew they were taking the active treatment but remained blind to the dose until after 1 year. After 1 year, patients were unblinded and continued to receive the same active dose as open-label treatment and were assessed every 8 weeks through 2 years and every 12 weeks from 2 years to 4 years.2 Starting at Week 128, patients whose signs and symptoms were not fully controlled and might improve further with an increase in dose as judged by the investigator, were updosed from 150 mg dose to 300 mg dose. At Week 208, 45 patients from the 150 mg dose arm had been updosed to 300 mg.3,4 A 75 mg arm was included in this study, but not shown and is not an approved dose.1 Study population was mixed: two-thirds of patients were anti-TNFα-naive and one-third were anti-TNFα-experienced (patients could have been exposed to up to 3 different TNFα inhibitors).2 At baseline, 44% of patients treated with COSENTYX were treated with concomitant MTX.2
FUTURE 51,6-8
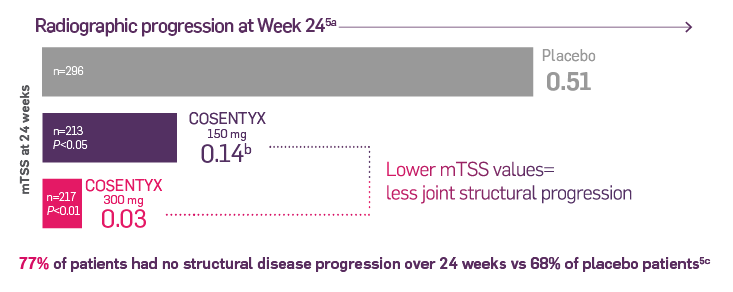
FUTURE 5 was a phase 3, multicenter, randomized, double-blind, placebo-controlled trial that is evaluating 996 adult patients with active PsA. Patients were randomized in 2:2:2:3 ratio to receive COSENTYX 150 mg without load (n=222), 150 mg with load (n=220), 300 mg with load (n=222), or placebo (n=332) subcutaneously at Weeks 0, 1, 2, and 3, followed by the same dose every 4 weeks. Patients who received placebo were rerandomized (1:1) to receive COSENTYX (either 150 mg or 300 mg every 4 weeks) based on responder status at Week 16 (nonresponders) or Week 24 (responders).6
The primary end point was the percentage of patients who achieved ACR20 response at Week 16.6 After Week 24, patients knew they were taking the active treatment but remained blind to the dose until after 1 year. Secondary end points included change in mTSS score at Week 24 from baseline, ACR50 response, proportion of patients with dactylitis and enthesitis, and overall safety and tolerability.6
Study population was mixed: more than two-thirds of patients were anti-TNF-α-naive and less than one-third were anti-TNF-α-experienced (patients could have been exposed to up to 3 different TNF-α inhibitors).6
ACR, American College of Rheumatology; DMARDs, disease-modifying antirheumatic drugs; mTSS, modified Total Sharp Score; MTX, methotrexate; NSAIDs, nonsteroidal anti-inflammatory drugs; PsA, psoriatic arthritis; TNF, tumor necrosis factor.
References
1. Cosentyx. Prescribing Information. Novartis Pharmaceuticals Corp.
2. Data on file. CAIN457F2312 Clinical Study Report. Novartis Pharmaceuticals Corp; October 2014.
3. Data on file. CAIN457F2312 (FUTURE 2): 3-Year Interim Study Report. Novartis Pharmaceuticals Corp; September 2017.
4. Data on file. CAIN457F2312 (FUTURE 2): 4-Year Interim Report. Novartis Pharmaceuticals Corp; 2018.
5. McInnes IB et al; for the FUTURE 2 Study Group. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomized, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;386:1137-1146. doi:10.1016/S0140-6736(15)61134-5
6. Data on file. CAIN457F2342 Clinical Study Report Interim Analysis at Week 24. Novartis Pharmaceuticals Corp; November 2017.
7. Mease P et al. Secukinumab improves active psoriatic arthritis symptoms and inhibits radiographic progression: primary results from the randomised, double-blind, phase III FUTURE 5 study. Ann Rheum Dis. 2018;77(6):890-897. doi:10.1007/s40265-014-0191-y
8. Data on file. CAIN457F2342 Clinical Study Report Interim Analysis - Week 52. Novartis Pharmaceuticals Corp; 2018.