Plaque psoriasis symptom relief
Help patients live with less compromise caused by their disease
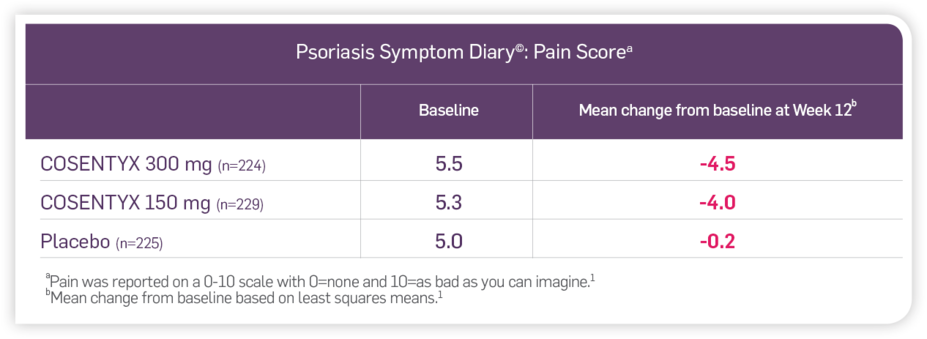
Patient-reported outcomes from the Psoriasis Symptom Diary© (PSD)1*
Among the subjects who chose to participate (39%) in assessments of patient-reported outcomes, improvements in signs and symptoms related to itching, pain, and scaling at Week 12 compared to placebo (ERASURE and FIXTURE studies) were observed using the PSD.2*
Reduction in psoriasis-related pain1
Least squares means change from baseline at Week 12 in other domains of the PSD1*
Less itching
COSENTYX 300 mg (n=224): -5.1 (mean score at baseline=6.4). COSENTYX 150 mg (n=229): -4.9 (mean score at baseline=6.5)
Placebo (n=225): -0.4 (mean score at baseline=6.1)
Less scaling
COSENTYX 300 mg (n=224): -5.2 (mean score at baseline=6.4). COSENTYX 150 mg (n=229): -4.8 (mean score at baseline=6.5)
Placebo (n=225): -0.3 (mean score at baseline=6.2)
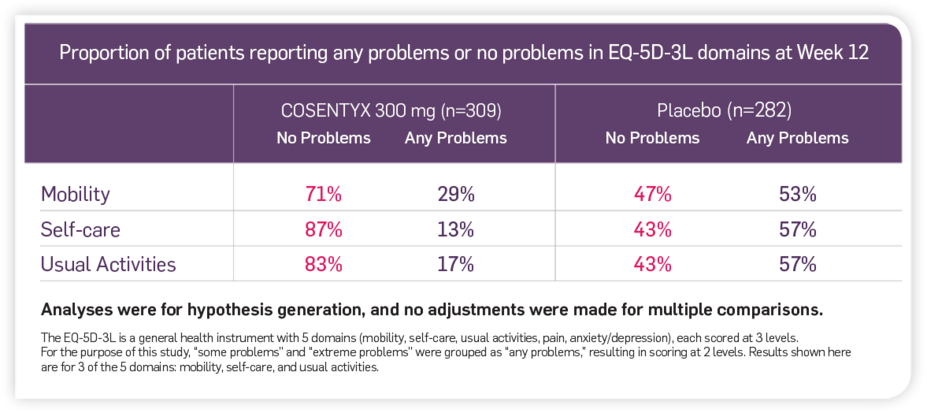
Patient-reported outcomes from the EQ-5D-3L
Improvement in mobility, self-care, and usual activities reported in an exploratory analysis3†
*The current analysis used pooled data from two randomized, double-blind, placebo-controlled, multicenter phase 3 trials (ERASURE and FIXTURE). The PSD was developed to capture the daily signs and symptoms of PsO reported by the patient, based on 16 items evaluating PsO-related characteristics that patients found important. Each item was rated on a scale of 0-10, with 0 indicating no effect and higher scores indicating worse effects of psoriasis. Analyses of the three PSD items—itching, pain, and scaling—were conducted using the 12-week induction period for subjects with a baseline and Week 12 PSD assessment. The results reported here focus on three psoriasis-related signs and symptoms—itching, pain, and scaling.1
†These results are from a pooled analysis of four phase 3 clinical trials (ERASURE, FIXTURE, FEATURE, and JUNCTURE), which included adult patients with moderate to severe PsO who were randomized to receive placebo or COSENTYX 300 mg and who reported problems with mobility, self-care, or usual activities at baseline. The objective of the study was to assess effect of COSENTYX treatment on mobility, self-care, and usual activities domains of the EQ-5D-3L questionnaire in patients with moderate to severe PsO who reported problems at baseline. The percentages of patients reporting problems in the EQ-5D-3L mobility, self-care, or usual activities domains were compared at Weeks 4, 8, and 12 between patients receiving placebo and those receiving COSENTYX 300 mg. Among the 282 patients receiving placebo and 309 receiving COSENTYX 300 mg, 172 and 180 reported any problems with mobility, 94 and 99 with self-care, and 214 and 254 with usual activities at baseline, respectively.3